Fair Medicine is an organisation that aims to introduce a new business model for the pharmaceutical industry. We believe that things can and should be done differently, but we do not wish to propagate too one-sided an opinion about the current market model. It has given us many good things over the years, but there are also problems with how new medications are currently financed. At the request of Fair Medicine, Gupta Strategists, a bureau specialised in research into health care expenses, has examined the costs of the current model utilised by the pharmaceutical industry. This study has resulted in five preliminary factors that have a disproportional influence on the price of medication:
- Financing costs: > 50% of the development costs
- High risk profile: results in high risk premium
- Large-scale, long-term trials: patients wait too long for medications
- High demands for return on investment, due to long investment term
- High failure rate: applies to many, but not all groups of medications
The pharmaceutical industry has brought many products to market, which benefit a large number of patients. After an initial period, during which patents protect a new product from competition through imitation, and investments can be earned back quickly, a second phase follows in which the market does its job and produces competition. This usually reduces prices to the level necessary to cover the costs of production and distribution, with a reasonable margin for healthy operations. That is, when all goes as it should.
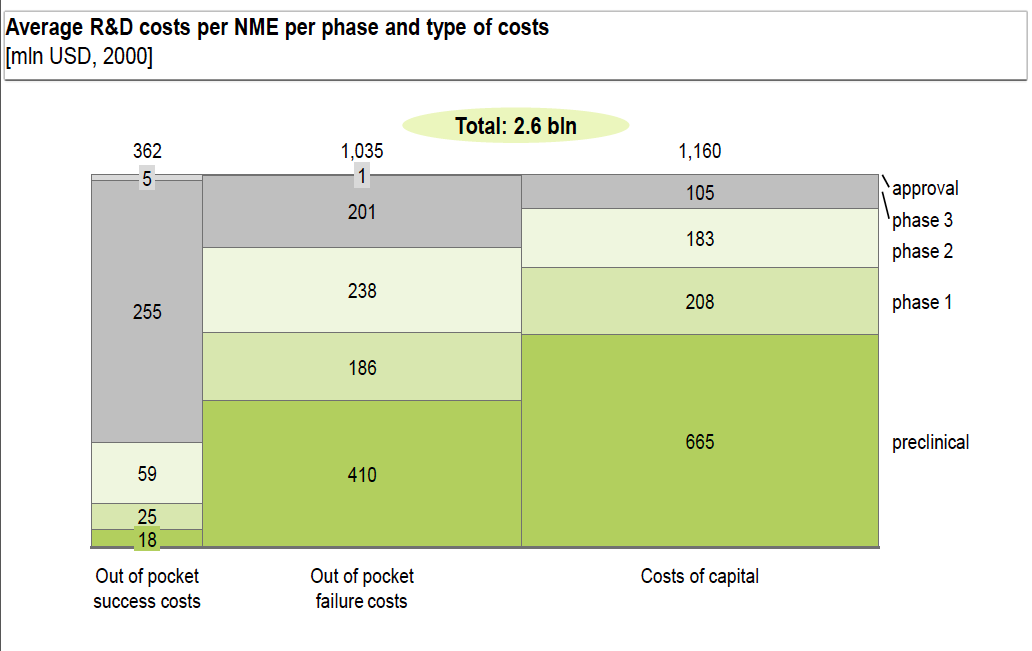 Unfortunately, we increasingly observe that this classic model is not working as intended. The industry often does not develop medications for small groups of patients, and when it does, they are usually offered for extremely high prices. These high prices in turn cause the authorities to decide not to cover the costs, or to do so only after long negotiations. Some recent examples include Spinraza and Orkambi. Both were initially rejected due to their high costs. This is to the detriment of everyone in the system, but especially to the patients, because they have to wait too long for medication to treat their conditions; medications which may not be covered by their insurance.
Unfortunately, we increasingly observe that this classic model is not working as intended. The industry often does not develop medications for small groups of patients, and when it does, they are usually offered for extremely high prices. These high prices in turn cause the authorities to decide not to cover the costs, or to do so only after long negotiations. Some recent examples include Spinraza and Orkambi. Both were initially rejected due to their high costs. This is to the detriment of everyone in the system, but especially to the patients, because they have to wait too long for medication to treat their conditions; medications which may not be covered by their insurance.
Fair Medicine was founded to develop business models that make it possible to develop products that would normally be overlooked by the classic model.
How does the analysis of cost drivers for pharmaceuticals help us to develop a new model that brings more products to the patient in less time, and for an affordable price? Thanks in part to the research by Gupta Strategists, which will publish its findings in the near future, Fair Medicine has identified the following priorities:
- The development of a new drug is a capital-intensive process. The analysis has shown that more than 50% of the development costs involve capital expenses – the money needed to finance the development. We must therefore search for opportunities to reduce these expenses.
- Pharmaceutical development is highly fragmented. The parties involved are only familiar with a part of the development process, and are not sufficiently involved in the other phases in the process; for example, doctors and patients are primarily users, and not co-developers of medications. Compensation is determined without knowing the actual development costs. For small patient groups, there is also often insufficient knowledge about the effectiveness of the drug. We must therefore combine more knowledge about the entire system, so that all of the parties involved can become better acquainted with one another’s contributions, and appreciate them.
- A limited group of investors and companies are able to bear the costs of financing the development of a new medication for the long term necessary, so we must give more parties – and other parties – access to the development of pharmaceuticals. This would involve interesting small- and medium-sized businesses, but also investors who would not normally be active in the pharmaceutical industry.
- Large-scale, long-term clinical studies are also drivers of the high costs for new pharmaceuticals. We must therefore study whether medications can be approved with smaller numbers of patients and shorter trails, for example when doing so would make it possible to make agreements on acceptable prices earlier, so that the system can compensate them faster, and limit the period of investment.
This is a short-but-sweet explanation, and there is much work to be done, but it provides a sufficient foundation for developing new models. To that end, Fair Medicine will collaborate with all of the stakeholders in the pharmaceutical development process. We call this new method the ‘coalition model’. We ask each party to adjust their classical role to a greater or lesser extent, in order to facilitate their collaboration in a coalition model. We are convinced that this will lead to a new stimulus for pharmaceutical development in the Netherlands, and its rapid spread to other countries as well.
To that end, we will soon meet with all of the stakeholders, in order to use their contributions to build even better models for cooperation.
Frans de Loos, April 2018.